how many electrons is one coulomb|Unit of charge (Coulombs) (video) : Clark • The magnitude of the electrical charge of one mole of elementary charges (approximately 6.022×10 , the Avogadro number) is known as a faraday unit of charge (closely . Tingnan ang higit pa My current McAfee Livesafe will be expiring soon. For the price of this activation key i bought this activation key. But there's a problem with the 25 digit product key. The product key were rip off. The clear protection seal is slid opened. Luckily i got it to work. I use the barcode to install the software.MARINES TV . Visit Marines TV for the latest videos from fellow Marines across the globe. Search by location and activity, find training videos, news stories and more
how many electrons is one coulomb,Every farad of capacitance can hold one coulomb per volt across the capacitor. One ampere hour equals 3600 C, hence 1 mA⋅h = 3.6 C. One statcoulomb (statC), the obsolete CGS electrostatic unit of charge (esu), is approximately 3.3356 × 10−10 C or about one-third of a nanocoulomb. Tingnan ang higit paThe coulomb (symbol: C) is the unit of electric charge in the International System of Units (SI). It is equal to the electric charge delivered by a 1 ampere current in 1 Tingnan ang higit paThe coulomb is named after Charles-Augustin de Coulomb. As with every SI unit named for a person, its symbol starts with an upper case letter (C), but when written in full, it . Tingnan ang higit pa

The SI defines the coulomb by taking the value of the elementary charge e to be 1.602176634×10 C, but was previously defined in . Tingnan ang higit paUnit of charge (Coulombs) (video) • The magnitude of the electrical charge of one mole of elementary charges (approximately 6.022×10 , the Avogadro number) is known as a faraday unit of charge (closely . Tingnan ang higit pa
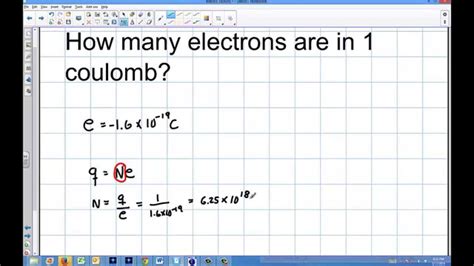
• The charges in static electricity from rubbing materials together are typically a few microcoulombs.• The amount of charge that travels through a lightning bolt is typically . Tingnan ang higit pa• Abcoulomb, a cgs unit of charge• Ampère's circuital law• Coulomb's law Tingnan ang higit paNamed for the 18th–19th-century French physicist Charles-Augustin de Coulomb, it is approximately equivalent to 6.24 × 10 18 electrons, with the charge of one electron, the .$$\because$$ 1 electron contains $$1.6 \times 10^{-19} C$$ of charge $$\therefore$$ 1 coulomb of charge is carried by $$\cfrac {1}{1.6 \times 10^{-19} } = 6.25 \times 10^{18}\ .
One coulomb is equal to the amount of charge from a current of one ampere flowing for one second. One coulomb is equal to the charge on 6.241 x 10 18 protons. The charge on 1 .
How many electrons does it take to make one coulomb of charge? The coulomb symbol is C, and according to the definition of a coulomb, each electron and proton has a charge magnitude of.
So to get one Coulumb worth of charge, we need so many electrons, so many and of course we can also write it in proper note to be 6.25 x 10, to the power 18 .The Coulomb is a ’derived’ unit. That is, its value depends on the basic units of the metric system, the meter, the kilogram, the second, and the Ampere. So one Coulomb is .How many electrons are there in a Coulomb? Solution. The coulomb ( C) in the International System of unit ( SI) is the standard unit of electric charge. It does not have any unit and .how many electrons is one coulomb Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal to 1.602176634 × 10−19 coulomb. In .F E is electric force, k is the Coulomb’s law constant, q 1 and q 2 are the charges, and r is the distance between the charges. The magnitude of the electric force between q 1 .
Faraday's number gives the charge held by 1 mole of electrons: 96,485 C. Therefore, 1 Coulomb is 1/96485 moles of electrons, or around 10−5 10 − 5 moles. Based on this, you can deduce that any corroded object of a reasonable size formed by passing at least 1 Coulomb of charge. Another point of comparison would be thinking about how . Transcript. Question 3 Page 200 Calculate the number of electrons constituting one coulomb of charge. We know that Charge on 1 Electron = 1.6 × 10−19 C 1 Electron = 1.6 × 10−19 C 1.6 × 10−19 C = 1 .
Electron charge is equal to the charge of an electron, and is the inverse of elementary charge, which is the magnitude of the charge of a proton. It is equal to 1.602176634×10−19 coulombs, per the 2019 SI redefinition of the coulomb. Electron charge can be abbreviated as e; for example, 1 electron charge can be written as 1 e.If 1 electron carries a charge of 1. 602 × 10-19 coulombs, what will be the charge of 1 mole of electrons? Q. which is bigger a coulomb or charge on an electron how many electronic charges form one coulomb of chargeOne coulomb consists of 6.24 × 10 18 natural units of electric charge, such as individual electrons or protons. From the definition of the ampere, the electron itself has a negative charge of 1.602176634 × 10 −19 coulomb. An electrochemical unit of charge, the faraday, is useful in describing electrolysis reactions, such as in metallic .
The total charge (C) transferred is the product of the current (A) and the time (t, in seconds): C = A × t (19.10.1) (19.10.1) C = A × t. The stoichiometry of the reaction and the total charge transferred enable us to calculate the amount of product formed during an electrolysis reaction or the amount of metal deposited in an electroplating .
One coulomb is equivalent to approximately 6.242 x 10^18 electrons. 6.24151 × 1018 electrons. The charge of 96,481 electrons; approximately counted as either 96,490 or 96,500 as per .Electrons and protons are not the only things that carry charge. Other particles (positrons, for example) also carry charge in multiples of the electronic charge. . 1.60 x 10-19 Coulombs. The Law of Conservation of Charge. The Law of conservation of charge states that the net charge of an isolated system remains constant.Compare the electrostatic force between an electron and proton separated by 0. 530 × 10 − 10 m 0. 530 × 10 − 10 m with the gravitational force between them. This distance is their average separation in a hydrogen atom. Strategy. To compare the two forces, we first compute the electrostatic force using Coulomb’s law, F = k | q 1 q 2 | r .How many electrons are in 2 coulombs? 2 C charge contains 1.25 × 10 19 electrons. This value is a product of the amount of Coulomb charge and the number of electrons in 1 C. How many electrons are in a microcoulomb? A microcoulomb (micro C) contains approximately 6.25 x 10 12 electrons. 1 micro C = 10-6 x 6.24 x 10 18 = 6.25 x 10 12
how many electrons is one coulomb Unit of charge (Coulombs) (video) If the charge on the electron is 1.6*10^-19 coulomb how many electrons should pass through a conductor in one sec to contribute 1ampere current. View Solution. Q5. how many electrons should pass through a conductor in 1 second to constitute 1 .The charges in Coulomb’s law are q 1 = q 2 = q ink drop, q 1 = q 2 = q ink drop, so the numerator in Coulomb’s law takes the form q 1 q 2 = q ink drop 2 q 1 q 2 = q ink drop 2. Inserting this into Coulomb’s law and solving for the distance r givesThe coulomb is defined as the quantity of electricity transported in one second by a current of one ampere. Named for the 18th–19th-century French physicist Charles-Augustin de Coulomb , it is approximately equivalent to 6.24 × 10 18 electrons , with the charge of one electron , the elementary charge , being defined as 1.602176634 × 10 −19 C.

The charge on the electron is 1.6 × 10^-19 C. The number of electrons need to be removed from a metal sphere of 0.05 m radius asked Jan 17, 2022 in Physics by Sanjana mali ( 37.9k points)Since one coulomb is equal to 6.2415E+18 electron charge, you can use this simple formula to convert: electron charge = coulombs × 6.2415E+18. The electric charge in electron charge is equal to the electric charge in coulombs multiplied by 6.2415E+18. For example, here's how to convert 5 coulombs to electron charge using the formula above.We would like to show you a description here but the site won’t allow us.A hydrogen atom consists of a single proton and a single electron. The proton has a charge of + e + e and the electron has − e − e . In the “ground state” of the atom, the electron orbits the proton at most probable distance of 5.29 × .
how many electrons is one coulomb|Unit of charge (Coulombs) (video)
PH0 · Unit of charge (Coulombs) (video)
PH1 · How many electrons are there in a Coulomb?
PH2 · How many electrons are there in 1 coulomb?
PH3 · Electrons in a Coulomb
PH4 · Electron charge
PH5 · Coulomb's law and electric force review (article)
PH6 · Coulomb Definition & Interaction
PH7 · Coulomb